Currently an informal name for the Standard Model
Chronological outline of the key theories:
- Maxwell's equations
- Schrödinger equation
- Date: 1926
- Numerical predictions:
- hydrogen spectral line, excluding finer structure such as 2p up and down split: en.wikipedia.org/wiki/Fine-structure_constant
- Dirac equation
- Date: 1928
- Numerical predictions:
- hydrogen spectral line including 2p split, but excluding even finer structure such as Lamb shift
- Qualitative predictions:
- Antimatter
- Spin as part of the equation
- quantum electrodynamics
- Date: 1947 onwards
- Numerical predictions:
- Qualitative predictions:
- Antimatter
- spin as part of the equation
There aren't any, it's useless:
The neutron temperature example is crucial: you just can't give the cross section of a target alone, the energy of the incoming beam also matters.
How to make a cloud chamber by Suzie Sheehy (2011)
Source. Tagged
Tagged
Most important application: produce X-rays for X-ray crystallography.
Note however that the big experiments at CERN, like the Large Hadron Collider, are also synchrotrons.
List of facilities: en.wikipedia.org/wiki/List_of_synchrotron_radiation_facilities
Predecessor to the synchrotron.
Nuclear physics is basically just the study of the complex outcomes of weak interaction + quantum chromodynamics.
Atomic Physics - An Historical Approach
. Source. By the British Department of Energy. Possibly: www.acmi.net.au/works/114589--atomic-physics-an-historical-approach/ which dates it 1945 - 1947.Side effect of the strong force that in addition to binding individual protons and neutrons as units, also binds different protons and neutrons to one another.
These are neutrons that have reached the thermal equilibrium according to the Maxwell-Boltzmann distribution after having bounced around many times without undergoing neutron capture.
Good fissile material is material that is able to absorb thermal neutrons and continue the reaction, because that's the type of neutron you end up getting the most of.
Some of the most notable ones:
- 1942: Chicago Pile-1: the first human-made nuclear chain reaction.
- 1943: X-10 Graphite Reactor: an intermediate step between the nuclear chain reaction prototype Chicago Pile-1 and the full blown mass production at Hanford site. Located in the Oak Ridge National Laboratory.
- 1944: B Reactor at the Hanford site produced the plutonium used for Trinity and Fat Man
Tagged
A nuclear reactor made to produce specific isotopes rather than just consume fissile material to produce electrical power. The most notably application being to produce Plutonium-239 for nuclear weapons from Uranium-238 being irradiated from Uranium-235-created fission.
Tagged
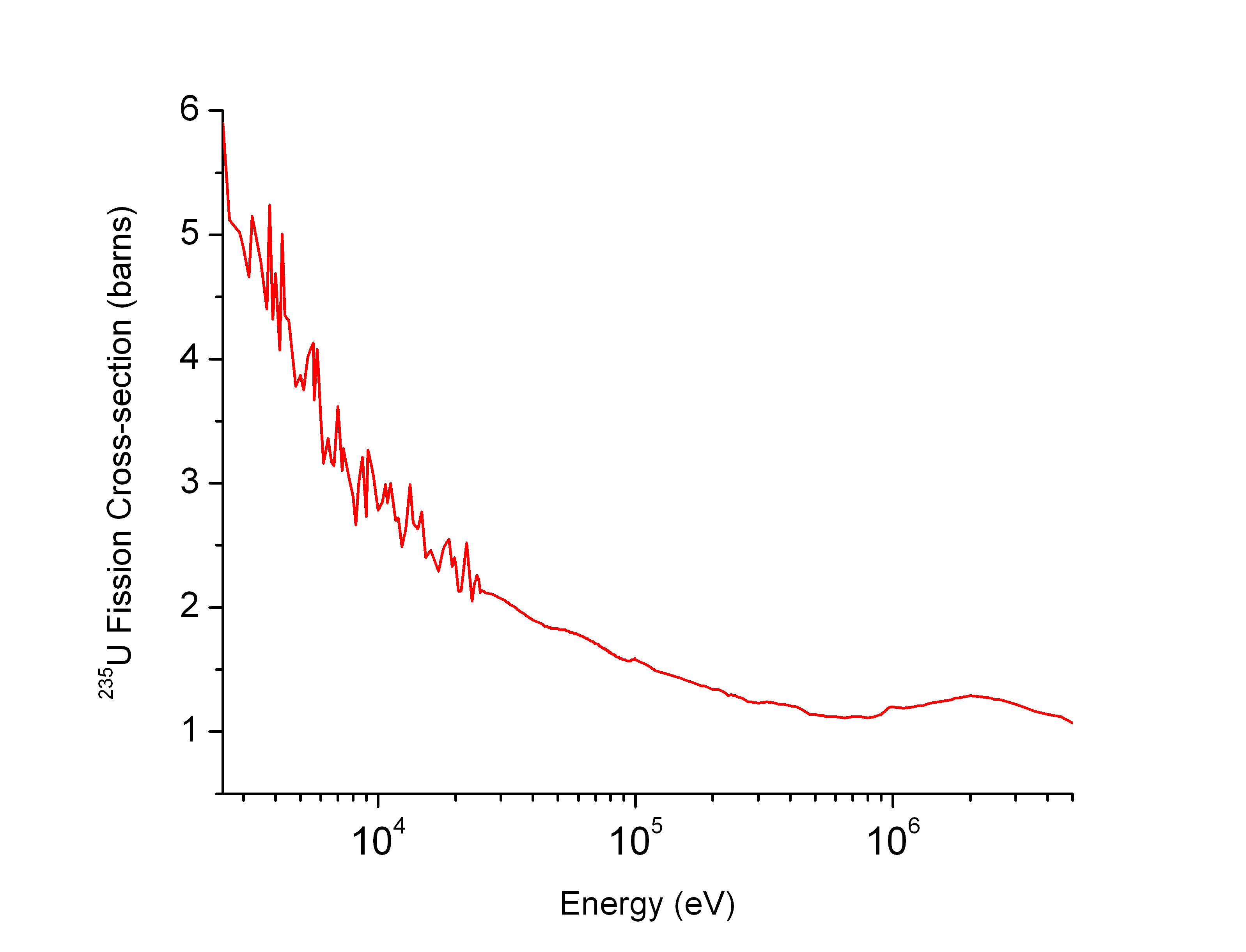
Uranium-235 neutron cross section as a function of neutron energy
. Source. Neutron cross section for various uranium isotopes
. Source. Ciro Santilli finds it interesting that radioactive decay basically kickstarted the domain of nuclear physics by essentially providing a natural particle accelerator from a chunk of radioactive element.
The discovery process was particularly interesting, including Henri Becquerel's luck while observing phosphorescence, and Marie Curie's observation that the uranium ore were more radioactive than pure uranium, and must therefore contain other even more radioactive substances, which lead to the discovery of polonium (half-life 138 days) and radium (half-life 1600 years).
Most of the helium in the Earth's atmosphere comes from alpha decay, since helium is lighter than air and naturally escapes out out of the atmosphere.
Wiki mentions that alpha decay is well modelled as a quantum tunnelling event, see also Geiger-Nuttall law.
As a result of that law, alpha particles have relatively little energy variation around 5 MeV or a speed of about 5% of the speed of light for any element, because the energy is inversely exponentially proportional to half-life. This is because:
- if the energy is much larger, decay is very fast and we don't have time to study the isotope
- if the energy is much smaller, decay is very rare and we don't have enough events to observe at all
- youtu.be/_f8zeEI0oys?t=796 George Gamow and Edward Condon proposed the quantum tunnelling explanation
- youtu.be/_f8zeEI0oys?t=1725 worked out example that predicts the half-life of polonium-210 based on its emission energy
They are stopped by:Therefore, alpha emitters are not too dangerous unless ingested.
- by a few centimeters of air
- a sheet of paper
- the skin
Uranium emits them, you can see their mass to charge ratio under magnetic field and so deduce that they are electrons.
Caused by weak interaction TODO why/how.
The emitted electron kinetic energy is random from zero to a maximum value. The rest goes into a neutrino. This is how the neutrino was first discovered/observed indirectly. This is well illustrated in a decay scheme such as Figure "caesium-137 decay scheme".
Most commonly known as a byproduct radioactive decay.
Their energy is very high compared example to more common radiation such as visible spectrum, and there is a neat reason for that: it's because the strong force that binds nuclei is strong so transitions lead to large energy changes.
A decay scheme such as Figure "caesium-137 decay scheme" illustrates well how gamma radiation happens as a byproduct of radioactive decay due to the existence of nuclear isomer.
Gamma rays are pretty cool as they give us insight into the energy levels/different configurations of the nucleus.
They have also been used as early sources of high energy particles for particle physics experiments before the development of particle accelerators, serving a similar purpose to cosmic rays in those early days.
But gamma rays they were more convenient in some cases because you could more easily manage them inside a laboratory rather than have to go climb some bloody mountain or a balloon.
The positron for example was first observed on cosmic rays, but better confirmed in gamma ray experiments by Carl David Anderson.
The following cobalt-60 diagrams suggest that some lines are clearly visible in specific nuclear reactions:
Also this one:
Example: Figure "caesium-137 decay scheme"
The half-life of radioactive decay, which as discovered a few years before quantum mechanics was discovered and matured, was a major mystery. Why do some nuclei fission in apparently random fashion, while others don't? How is the state of different nuclei different from one another? This is mentioned in Inward Bound by Abraham Pais (1988) Chapter 6.e Why a half-life?
The term also sees use in other areas, notably biology, where e.g. RNAs spontaneously decay as part of the cell's control system, see e.g. mentions in E. Coli Whole Cell Model by Covert Lab.
Neon isotope line split photograph by J. J. Thomson
. Source. J. J. Thomson took this picture in 1912:There can, therefore, I think, be little doubt that what has been called neon is not a simple gas but a mixture of two gases, one of which has an atomic weight about 20 and the other about 22. The parabola due to the heavier gas is always much fainter than that due to the lighter, so that probably the heavier gas forms only a small percentage of the mixture.
Tagged
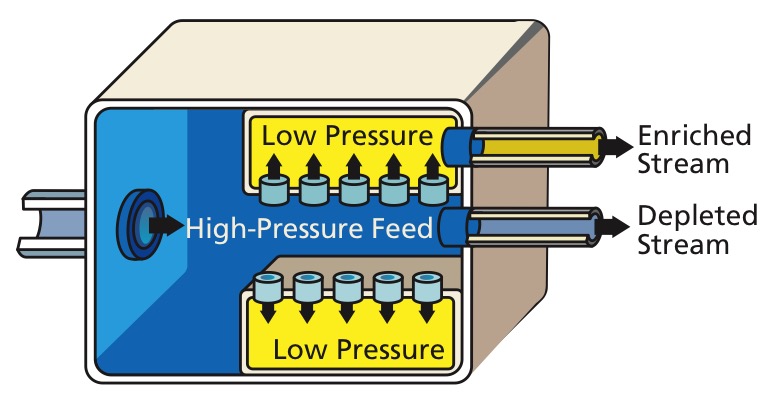
This isotope separation method was the first big successful method, having been used in the Manhattan Project, notably in the K-25 reactor.
This method was superseded by the more efficient gas centrifuges.
Gaseous diffusion diagram
. Source. Ciro Santilli once visited the chemistry department of a world leading university, and the chemists there were obsessed with NMR. They had small benchtop NMR machines. They had larger machines. They had a room full of huge machines. They had them in corridors and on desk tops. Chemists really love that stuff. More precisely, these are used for NMR spectroscopy, which helps identify what a sample is made of.
Basically measures the concentration of certain isotopes in a region of space.
Introduction to NMR by Allery Chemistry
. Source. - only works with an odd number of nucleons
- apply strong magnetic field, this separates the energy of up and down spins. Most spins align with field.
- send radio waves into sample to make nucleons go to upper energy level. We can see that the energy difference is small since we are talking about radio waves, low frequency.
- when nucleon goes back down, it re-emits radio waves, and we detect that. TODO: how do we not get that confused with the input wave, which is presumably at the same frequency? It appears to send pulses, and then wait for the response.
How to Prepare and Run a NMR Sample by University of Bath (2017)
Source. This is a more direct howto, cool to see. Uses a Bruker Corporation 300. They have a robotic arm add-on. Shows spectrum on computer screen at the end. Shame no molecule identification after that!Proton Nuclear Magnetic Resonance by Royal Society Of Chemistry (2008)
Source. This video has the merit of showing real equipment usage, including sample preparation.
Says clearly that NMR is the most important way to identify organic compounds.
- youtu.be/uNM801B9Y84?t=41 lists some of the most common targets, including hydrogen and carbon-13
- youtu.be/uNM801B9Y84?t=124 ethanol example
- youtu.be/uNM801B9Y84?t=251 they use solvents where all protium is replaced by deuterium to not affect results. Genius.
- youtu.be/uNM801B9Y84?t=354 usually they do 16 radio wave pulses
NMR spectroscopy visualized by ScienceSketch
. Source. 2020. Decent explanation with animation. Could go into a bit more numbers, but OK.Published as A New Method of Measuring Nuclear Magnetic Moment.
Bibliography:
Tagged
The equation is simple: frequency is proportional to field strength!
Used to identify organic compounds.
Seems to be based on the effects that electrons around the nuclei (shielding electrons) have on the outcome of NMR.
So it is a bit unlike MRI where you are interested in the position of certain nuclei in space (of course, these being atoms, you can't see their positions in space).
What's Nuclear Magnetic Resonance by Bruker Corporation (2020)
Source. Good 3D animations showing the structure of the NMR machine. We understand that it is very bulky largely due to the cryogenic system. It then talks a bit about organic compound identification by talking about ethanol, i.e. this is NMR spectroscopy, but it is a bit too much to follow closely. Basically the electron configuration alters the nuclear response somehow, and allows identifying functional groups.How does an MRI machine work? by Science Museum (2019)
Source. The best one can do in 3 minutes perhaps.How MRI Works Part 1 by thePIRL (2018)
Source. - youtu.be/TQegSF4ZiIQ?t=326 the magnet is normally always on for the entire lifetime of the equipment!
- youtu.be/TQegSF4ZiIQ?t=465 usage of non-ionizing radiation (only radio frequencies) means that it is very safe to use. The only dangerous part is the magnetic field interacting with metallic objects.
Dr Mansfield's MRI MEDICAL MARVEL by BBC
. Source. Broadcast in 1978. Description:Tomorrow's World gave audiences a true world first as Dr Peter Mansfield of the University of Nottingham demonstrated the first full body prototype device for Magnetic resonance imaging (MRI), allowing us to see inside the human body without the use of X-rays.
This is a good book, it gives a summary of biographies, and a reasonable description of the main ideas, with many illustrations. Each subject is not presented in incredible detail, but it is a good overview of events.
Free borrow on the Internet Archive: archive.org/details/inwardboundofmat0000pais/page/88/mode/2up
The book unfortunately does not cover the history of quantum mechanics very, the author specifically says that this will not be covered, the focus is more on particles/forces. But there are still some mentions.
Tagged
Some light YouTube channels, good for the first view, but which don't go into enough detail to truly show the subject's beauty:
Always a bit too much on the superficial side, but sometimes OK, 5-10 minute videos.
These feel good. Targeted at upper undergrads, so he says he holds back on some stuff, but gives a good level of detail for people who have a life.
Too many fun skit videos for Ciro Santilli's taste, but does have some serious derivations in quantum electrodynamics.
Covers some specific hardcore subjects, notably quantum electrodynamics, in full mathematical detail, e.g.: "Quantum Field Theory Lecture Series" playlist: www.youtube.com/playlist?list=PLSpklniGdSfSsk7BSZjONcfhRGKNa2uou
As of 2020 Dietterich was a condensed matter PhD candidate or post-doc at the University of Minnesota Twin Cities, and he lives in Minnesota, sources:
Unfortunately the channel is too obsessed with mathematical detail (which it does amazingly), and does not give enough examples/application/intuition, which is what would be useful to most people, thus falling too much on the hardcore side of the missing link between basic and advanced.
This channel does have on merit however: compared to other university courses, it is much more direct, which might mean that you get to something interesting before you got bored to death, Section "You can learn more from older students than from faculty" comes to mind.
Videos generally involves short talks + a detailed read-through of a pre-prepared PDF. Dietterich has refused however giving the PDF or LaTeX source as of 2020 on comments unfortunately... what a wasted opportunity for society. TODO find the comment. Sam, if you ever Google yourself to this page, let's make a collab on OurBigBook.com and fucking change education forever man.
Full name as shown in channel content: Samuel Dietterich. Other accounts:
The Ultimate Goal Of My YouTube Channel by Dietterich Labs (2020)
Source. In this video Dietterich gives his ideal for the channel. Notably, he describes how the few experimental videos he has managed to make were done in a opportunistic way from experiments that were happening around him. This resonated with Ciro Santilli's ideas from videos of all key physics experiments.Sam Dietterich interview by Dietterich Labs (2022)
Source. TODO find patience to watch and summarize key points.The Sting Of Soft Corruption: My College Experience by Dietterich Labs
. Source. Academia is broken video.Almost always too short superficial where it matters unfortunately, as usual.
Those guys are really good, Ciro Santilli especially enjoyed their quantum mechanics playlist: www.youtube.com/playlist?list=PL193BC0532FE7B02C
The quantum electrodynamics one was a bit too slow paced for Ciro unfortunately, too much groundwork and too little results.
Accompanying website with a tiny little bit of code: viascience.org/what.html
TODO: authors and their affiliation.
Videos licensed as CC BY-SA, those guys are so good.
These videos can give some geometric insight and do have their value.
But they are sometimes too slow, there are never any mention of experiments, just "the truth".
And when things get "mathy", it sticks to a more qualitative view which may not be enough.
Very over the top with sexy demons and angels making appearances, and has some classic aesthetic artistic value :-)
Eugene's background: www.quora.com/Who-is-Eugene-Khutoryansky/answer/Ciro-Santilli
Publishes through the Fermilab YouTube channel under the playlist "Fermilab - Videos by Don Lincoln"
Some insights, but too much on the popular science side of things.
 Ciro Santilli
Ciro Santilli OurBigBook.com
OurBigBook.com